Long Genes as an Aging Mechanism?
The Central Dogma of Biology.
You all know that DNA gets transcribed to RNA which gets translated to protein, to get coded information into action in the body. This is so basic, that we call it the The Central Dogma of Biology - the flow of genetic information within a biological system is that “DNA makes RNA, and RNA makes protein”. Here are some review articles if you haven’t thought about this for awhile.
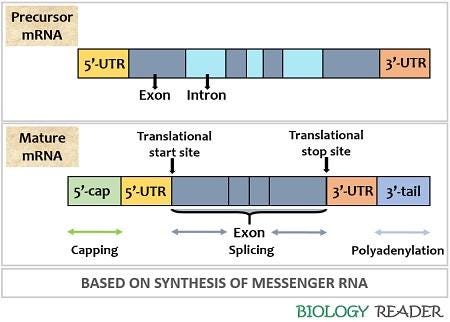
Obviously, it takes longer and takes more energy to transcribe a long stretch of DNA into RNA, rather than a short stretch. And in eukaryotes, there are extra steps needed like splicing, capping, and adding adenosines to the tail or mRNA (so called Maturing). A large gene might have more than one section that needs to be spliced-out in creating the mature mRNA. If the DNA is damaged near one of these splice sites, it could derail the process of creating a good mature mRNA.
Background: In eukaryotes, the mRNA is processed after transcription by splicing out part of it (introns) and connecting the remaining parts (exons) and adding adenosines to the end (polyadenylation). Then you have a 'mature' mRNA that can be used to create proteins. What you mainly need to know for this article is that you can't make a proper protein from mRNA until you do these steps correctly. A new phenomenon called Gene Length-Dependent Transcription Decline (GLTD), has been observed in aging organisms across various species and across various tissues. GLTD is characterized by a negative correlation between gene length and expression levels. [1] In other words, longer genes tend to be expressed less as organisms age.
Key points.
Key things that we know about this effect:
-It is universal. It is observed across diverse species and diverse tissues.
-GLTD is linked to aging: It's observed in normal aging and accelerated aging conditions like Alzheimer's disease and premature aging mouse models.
-Accumulation of DNA damage, particularly in long genes, is a key driver of GLTD. This damage can stall RNA polymerase, leading to reduced transcription.
-Extremely long genes, especially those involved in neuronal and synaptic functions, are particularly susceptible to damage and GLTD.
-It doesn’t appear to be a root cause for aging, but an important fast follower on DNA damage. So fixing it could be at the DNA damage (prevention /repair/additional duplicate gene), or the RNA point
-Fixing the epigenetic age of a cell should not fix this issue, since epigenetic state only controls whether or not a gene is going to be expressed. It doesn’t affect how WELL the gene gets expressed. In other words, this appears to be a separate aging mechanism that Yamanaka Factors and epigenetic reversal of age, will not fix.
Reasons for GLTD?
Here’s a summary of some possible causes of GLTD:
1. DNA Damage Accumulation
Oxidative Damage and Other DNA Lesions: Over time, DNA accumulates various forms of damage, such as oxidative lesions and double-strand breaks. These damages can block the progression of RNA polymerase, the enzyme responsible for transcribing DNA into RNA.
Transcription-Blocking Lesions: DNA damage that directly interferes with the transcription machinery tends to have a more pronounced effect on longer genes. Since longer genes require more time for transcription, they are more likely to encounter DNA damage during the transcription process.
2. Deficiencies in DNA Repair Mechanisms
Reduced Efficiency of Repair Pathways: As organisms age, the efficiency of DNA repair pathways (such as nucleotide excision repair and base excision repair) declines. This diminished capacity to repair DNA damage exacerbates the transcription decline, particularly for long genes.
Transcription-Coupled Repair (TCR): Although TCR is a mechanism that preferentially repairs genes that are actively being transcribed, its efficiency also decreases with age, contributing to GLTD.
3. Slowing of Transcriptional Elongation
Impaired RNA Polymerase Progression: With age, the rate at which RNA polymerase moves along the DNA strand can slow down. This is particularly problematic for long genes because the longer transcription takes, the higher the chance that the polymerase will encounter DNA damage or other obstacles.
Chromatin Structure Changes: Age-related changes in chromatin structure, such as increased heterochromatin formation, can impede the movement of RNA polymerase, further contributing to the decline in transcriptional elongation for long genes.
4. Decline in Cellular Resources
Depletion of Transcription Factors and Cofactors: As organisms age, there may be a decrease in the availability of essential transcription factors and cofactors needed for efficient transcription. This depletion disproportionately affects long genes, which require more of these resources for complete transcription
How can you fix GLTD?
Obviously, one fix would be to prevent DNA damage or fix DNA damage better. Many organisms have mechanisms to do that, which we are already familiar with. And organisms with better DNA damage control do in fact live longer. [4]
For example, the cell that has the DNA damage can be made to kill itself (apoptosis), or the cell enters senescence and stops dividing, and/or the immune system removes the DNA damaged cell.
One could speculate that lower energy levels in older cells could have an impact or having fewer of the transcription factors that are needed for the transcription process.
But Aptah Bio believes they have a drug to fix this, at the RNA level. [2] Aptah Bio's RNA WiCo™ technology is reported as being “RNA Rejuvenation Technology” [2]
What does that mean?
Pre-mRNA splicing and polyadenylation are crucial steps in normal eukaryotic mRNA processing (like humans do). And U1 snRNP is part of this splicing process, to cut out the unneed RNA and connect the exons (needed parts) together. This happens a lot in our cells, and it must be accurate, so that accurate proteins can be created. If this begins to malfunction in aging, then large proteins will be affected more than smaller ones, but many genes could be affected. This happens in aging.
If aging is caused by inaccurate splicing, and this drug can correct that for all genes, then this type of aging mechanism should be correctable in older cells.
This drug works by enhancing the function of U1-snRNP which can create toxic proteins via incorrect splicing [5] leading to the expression of widespread full-length 3′UTRs. By correcting the U1-snRNP behaviour, their product can create overall RNA quality control.
Next?
I am unclear where this research goes. I don’t believe that there can be a magic bullet for this, because there are so many moving parts, and so many separate issues in this process. I do believe that this drug could fix some of the issues. And I believe that this is an important area of research because it appears that there are ways to make splicing more accurate, even in the face of damage.
Likewise, better DNA damage prevention and repair could help this process. Or killing off the DNA damaged cells and depending on replacing the whole cells would be a good method of dealing with DNA damage, if you have sufficient stem cell activity to depend on replacement, and if you have a good way to kill these cells (think senolytics, apoptosis, immune system, mitoautophagy).
But with Altos Labs, Calico, and Life Biosciences making progress on epigenetic cellular rejuvenation, it is not too early to start thinking about what would need done afterwards - what aging processes will not be addressed by epigenetic rejuvenation?
Certainly, this is one of those.**
References
[1] Soheili-Nezhad S, Ibáñez-Solé O, Izeta A, Hoeijmakers JHJ, Stoeger T. Time is ticking faster for long genes in aging. Trends Genet. 2024 Apr;40(4):299-312. doi: 10.1016/j.tig.2024.01.009. Epub 2024 Mar 21. PMID: 38519330; PMCID: PMC11003850. https://pubmed.ncbi.nlm.nih.gov/38519330/
[2] https://www.aptah-bio.com/
[3] https://pubmed.ncbi.nlm.nih.gov/33979534/
[4] https://www.nature.com/articles/s41586-021-03307-7
[5] https://www.aptah-bio.com/
[6] https://biologyreader.com/messenger-rna.html
** Some of you may be clever enough to think that turning off damaged genes via epigenetic mechanisms will help this issue. And it will, but it will Not help those genes that are damaged and really need to be turned On for this cell type.
Disclaimer: I am not a doctor. Nor do I play one on TV. The information on this blog is for informational purposes only and is not intended to be medical advice. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional for medical advice and treatment. The author of this blog is not a licensed medical professional and does not claim to be an expert in any field of medicine.
Disclaimer. Last updated November 17, 2024